S-(2-aminoethyl)isothiourea dihydrobromide
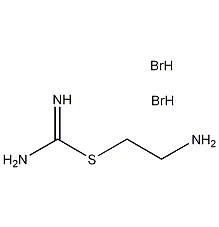
Structural formula
| Business number | 0177 |
|---|---|
| Molecular formula | C3H9N3S·2HBr |
| Molecular weight | 281.02 |
| label |
2-(2-Aminoethyl)isothiourea dihydrobromide, Bromide-S-(aminoethyl)isothiourea hydrobromide, 2-(2-Aminoethyl)-2-thiopseudourea dihydrobromide, AET, β-Aminoethylisothiuronium bromide hydrobromide, Antirad, Antiradon, Carbamimidothioic acid-2-aminoethyl ester dihydrobromide, Surrectan |
Numbering system
CAS number:56-10-0
MDL number:MFCD00037011
EINECS number:200-257-0
RTECS number:UM0175000
BRN number:3911163
PubChem number:24278229
Physical property data
1. Properties: White crystal, easy to deliquify and separate and close rings to synthesize isomers.
2. Density (g/mL, 25/4℃): Undetermined
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 194-195℃
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 5.2kPa) : Undetermined
7. Refractive index: Undetermined
8. Flash point (ºC): Undetermined
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
12 . Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
p>
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Easily soluble in water, soluble in ethanol
Toxicological data
1. Acute toxicity: rat intraperitoneal LD50: 288mg/kg; rat intravenous LD50: 85mg/kg; mouse oral LD50: 815mg/kg; mouse intraperitoneal LD50: 400mg/kg; mouse subcutaneous LD50: 242mg/kg ; Mouse intravenous LD50: 85400ug/kg; Oral LD50: 177mg/kg; Dog intraperitoneal LD50: 113mg/kg; Rabbit intraperitoneal LD50: 236mg/kg; Guinea pig intraperitoneal LD50: 356mg/kg2, other multi-dose toxicity: mice Abdominal TDLo: 5474ng/kg/40D-I; dog abdominal TDLo: 415mg/kg/15D-I3, mutagenicity: Micronucleus test: mouse abdominal cavity: 200mg/kg
Ecological data
None
Molecular structure data
None
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP):
2. Number of hydrogen bond donors: 3
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 3
5. Number of tautomers:
6. Topological molecular polar surface area (TPSA): 75.9
7. Number of heavy atoms: 9
8. Surface charge: 0
9. Complexity: 63.2
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters : 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 3
Properties and stability
1. Easy to deliquesce.
Storage method
Store sealed and dry.
Synthesis method
(1) After bromination of aminoethanol to obtain bromoethylamine hydrobromide, it is condensed with thiourea to obtain: Bromination: Put hydrobromic acid into the reaction tank, after cooling, add aminoethanol dropwise with stirring, within 30 minutes After the dropwise addition is completed, 85% of the input amount of hydrobromic acid is evaporated, and the evaporation is completed in about 20 hours. After cooling the concentrated solution to 70-80°C, put it into pre-frozen acetone, then cool it to below 5°C to crystallize, filter, wash with cold acetone, and dry to obtain bromoethylamine hydrobromide. Melting point 165℃. Condensation: Mix and stir isopropyl alcohol and thiourea, heat to 70°C, quickly add bromoethylamine hydrobromide, raise the internal temperature to 82°C, and react for 40 minutes. Cool to below 10°C and filter, wash with cold isopropyl alcohol, then wash with a small amount of ethyl acetate, and dry to obtain the crude product of Ke Naomi. Add 4.75 times methanol (V/V) and 0.02 times activated carbon to the crude product, stir and heat to reflux for 15-20 minutes. Filter while hot, cool, and add 4.28 times the amount of crude product ether (V/V) after half an hour. Continue to cool, filter and dry after complete crystallization to obtain the finished product. The total yield is 56-58% (based on aminoethanol). (2) Use cycloethylamine, hydrobromic acid, and thiourea as raw materials to synthesize: first mix thiourea and bromohydric acid, add cycloethylamine dropwise below 150°C, after the reaction is completed, add activated carbon to filter, and then reduce the pressure After dehydration, crystals will precipitate when the temperature is below 50°C. Filter and wash twice with absolute ethanol to obtain the finished product.
Purpose
Used in organic synthesis, enzyme activator, radiation sickness prevention, and free radical detoxifier. As a drug, this product can promote brain cell metabolism, increase the utilization of carbohydrates, and improve central excitability. It can quickly restore brain function to traumatic coma patients and has the effect of counteracting central depressant drugs. It is suitable for traumatic coma, coma caused by cardiovascular diseases, carbon monoxide poisoning, barbiturate and tranquillizer poisoning, radiation damage and cerebral hypoxia, etc.
extended-reading:https://www.bdmaee.net/jeffcat-zf-22/extended-reading:https://www.newtopchem.com/archives/462extended-reading:https://www.bdmaee.net/hydroxy-nnn-trimethyl-1-propylamine-formate-cas62314-25-4-catalyst-tmr-2/extended-reading:https://www.morpholine.org/polyurethane-catalyst-1028/extended-reading:https://www.newtopchem.com/archives/45231extended-reading:https://www.bdmaee.net/pc-cat-dmdee-catalyst-22-dimorpholino-diethyl-ether/extended-reading:https://www.newtopchem.com/archives/43904extended-reading:https://www.newtopchem.com/archives/category/products/page/108extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/TIB-KAT-129.pdfextended-reading:https://www.newtopchem.com/archives/40226


