Methyl mercaptan
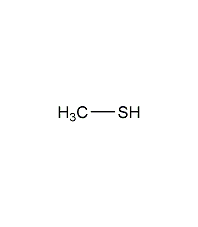
Structural formula
| Business number | 01HN |
|---|---|
| Molecular formula | CH3SH |
| Molecular weight | 48 |
| label |
Methanethiol, Mercaptan methylique, Methyl thioalcohol, aliphatic sulfur compounds, Alcohols |
Numbering system
CAS number:74-93-1
MDL number:MFCD00004866
EINECS number:200-822-1
RTECS number:PB4375000
BRN number:1696840
PubChem ID:None
Physical property data
1. Characteristics: colorless gas with unpleasant odor. [1]
2. Melting point (℃): -123.1[2]
3. Boiling point (℃): 6[3]
4. Relative density (water = 1): 0.9[4]
5. Relative vapor Density (air=1): 1.66[5]
6. Saturated vapor pressure (kPa): 202 (26.1℃)[6]
7. Heat of combustion (KJ/mol): -1235.0[7]
8. Critical temperature (℃): 196.8[8]
9. Critical pressure (MPa): 7.23[9]
10. Octanol/water partition coefficient: 0.65 [10]
11. Flash point (℃): <-17.78[11]
12. Ignition temperature (℃) :325[12]
13. Explosion upper limit (%): 21.8[13]
14. Explosion lower limit ( %): 3.9[14]
15. Solubility: Insoluble in water, soluble in ethanol, ether, petroleum naphtha, etc. [15]
Toxicological data
1. Acute toxicity: rat inhalation LC50: 675 ppm; mouse inhalation LC50: 6530 ug/m3/2H; mammals – species unknown, route unknown LD50: 60670 ug/kg;
2 , mutagenicity: loss and non-disjunction of sex chromosomes in Drosophila inhalation: 99 pph/6M (continuous);
3. Acute toxicity[16]
LD50: 60.67mg/kg, mammal (unknown species)
LC50: 1325mg/m3 (675ppm ) (rat inhalation); 6.5mg/m3 (mouse inhalation, 2h)
Ecological data
1. Ecotoxicity[17] LC50: 0.55~0.9mg/L (96h) (salmon)
2. Biodegradability No data yet
3. Non-biodegradability[18] In the air, when the concentration of hydroxyl radicals is 5.00×105/cm3, the degradation half-life is 11.7h (theoretical).
4. Other harmful effects[19] This substance is harmful to the environment. Special attention should be paid to water and air pollution.
Molecular structure data
1. Molar refractive index: 14.57
2. Molar volume (cm3/mol): 59.0
3. Isotonic specific volume (90.2K ): 123.7
4. Surface tension (dyne/cm): 19.2
5. Polarizability (10-24cm3): 5.77
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 0.5
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 1
7. Number of heavy atoms: 2
8. Surface charge: 0
9. Complexity: 2
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stability[20] Stable
2. Incompatible substances[21] Strong oxidants, halogens, acids
3. Conditions to avoid contact[22] Humid air
4. Hazards of aggregation[23] No aggregation
Storage method
Storage Precautions[24] Store in a cool, ventilated warehouse dedicated to flammable gases. Keep away from fire and heat sources. The storage temperature should not exceed 30℃. Keep container tightly sealed. They should be stored separately from oxidants, acids, and halogens, and avoid mixed storage. Use explosion-proof lighting and ventilation facilities, and prohibit the use of mechanical equipment and tools that are prone to sparks. The storage area should be equipped with leakage emergency response equipment.
Synthesis method
The gas phase synthesis method of methanol and hydrogen sulfide uses activated alumina as the main catalyst, the reaction temperature is 280-450°C, and the reaction pressure is 0.74-0.25MPa. Using this method to produce methyl mercaptan, the yield based on methanol can exceed 64-70%. The method of producing mercaptans by using halogenated hydrocarbons and sulfhydryl bases (or thioureas), olefins and hydrogen sulfide can also be used in industry. In smaller-scale production, the method of reacting thiourea with dimethyl sulfate and then adding alkali hydrolysis can be used. Add water to the reaction pot, add thiourea while stirring, and add dimethyl sulfate dropwise. When the temperature naturally reaches 80-90°C, then heat to 120°C and react until the material becomes viscous. After discharging and cooling, the methyl thiourea salt filter cake is obtained, and then the temperature is controlled at 50-60°C, and the sodium hydroxide solution is added dropwise to the methyl isothiourea sulfate to generate methyl mercaptan.
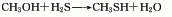
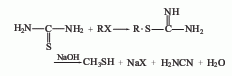
Purpose
Used in the synthesis of western medicines, pesticides, methionine, and as an odor enhancer for odorless gases. [25]
extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/38-7.jpgextended-reading:https://www.morpholine.org/k-15/extended-reading:https://www.newtopchem.com/archives/39151extended-reading:https://www.newtopchem.com/archives/44968extended-reading:https://www.bdmaee.net/delay-catalyst-a-300/extended-reading:https://www.bdmaee.net/nt-cat-16-catalyst-cas280-57-9-newtopchem/extended-reading:https://www.newtopchem.com/archives/44983extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/-NEM-Niax-NEM-Jeffcat-NEM.pdfextended-reading:https://www.bdmaee.net/dioctyltin-dilaurate-dotdl/extended-reading:https://www.newtopchem.com/archives/39156


