Benzyltriethylammonium chloride
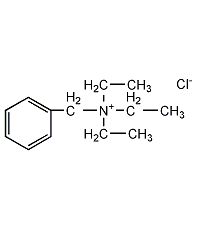
Structural formula
| Business number | 017J |
|---|---|
| Molecular formula | C13H22ClN |
| Molecular weight | 227.78 |
| label |
Triethylbenzylammonium chloride, TEBA, Fungicide |
Numbering system
CAS number:56-37-1
MDL number:MFCD00011824
EINECS number:200-270-1
RTECS number:BO8275000
BRN number:3574984
PubChem number:24848415
Physical property data
1. Properties: white crystal. Hygroscopic.
2. Density (g/mL, 25/4℃): Undetermined
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 190℃ (decomposition)
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 5.2kPa ): Not determined
7. Refractive index: Not determined
8. Flash point (ºC): Not determined
9. Specific rotation (º) : Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion Upper limit (%, V/V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Easily soluble in water.
Toxicological data
1. Acute toxicity: rat oral LD50: 2219mg/kg; mouse intravenous LC50: 18mg/kg
Ecological data
None
Molecular structure data
None
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 5
5. Number of tautomers: none
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 15
8. Surface charge: 0
9. Complexity: 135
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 2
Properties and stability
Cannot be blended with anions.
Storage method
This product should be kept sealed and dry.
Synthesis method
1. Add benzyl chloride, triethylamine, and acetone to the reaction pot and reflux at 63-64°C for 8 hours. Slowly lower to 15°C, filter, wash the filter cake with acetone, and dry to obtain TEBA. The yield is 68.9%. The reaction formula is as follows:
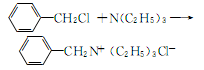
2.Place 346.5 grams of triethylamine, 413.5 grams of chlorine and ethyl acetate in 238.6 grams of dimethylformamide (DMF) and reflux for 1 hour, add 300 Gram benzene,precipitates ammonium salt. Suction filter, wash with benzene, and vacuum dry to obtain 648 grams, purity 98.1%
You can also place 25 grams of triethylamine and 30 grams of benzyl chloride in 120 grams of dichloroethane and reflux for 2 hours to obtain 52.6 grams Products
Purpose
1. Alkylation reaction catalyst. Phase transfer catalyst. Multi-substituted cyclopropanes are synthesized through phase transfer catalytic Michael addition reaction.
2.This product is used as a fungicide.
3.Purpose phase transfer catalyst. Used in nucleophilic substitution, carbene reaction and alkylation reactions such as C-alkylation, N-alkylation, 0-alkylation and S-alkylation.
extended-reading:https://www.bdmaee.net/fomrez-ul-29-catalyst-octylmercaptan-stannous-momentive-2/extended-reading:https://www.bdmaee.net/cas-23850-94-4-2/extended-reading:https://www.newtopchem.com/archives/970extended-reading:https://www.bdmaee.net/pc-cat-t9-catalyst-nitro/extended-reading:https://www.bdmaee.net/wp-content/uploads/2021/05/137-2.jpgextended-reading:https://www.newtopchem.com/archives/44561extended-reading:https://www.bdmaee.net/bismuth-isooctanoate-cas67874-71-9-2-ethylhexanoic-acid-bismuth/extended-reading:https://www.cyclohexylamine.net/nn-dicyclohexylmethylamine/extended-reading:https://www.bdmaee.net/wp-content/uploads/2020/07/86.jpgextended-reading:https://www.newtopchem.com/archives/40343


